Trial design
If you are asked to take part in a phase 2 or phase 3 study, there are a number of terms which you may hear.
Controlled trials
In most trials, one group of patients will have the trial treatment and one group will have the standard treatment. The people having the trial treatment are known as the trial group and the people having the standard treatment are known as the control group. Without a control group it is impossible to measure how much of the improvement in the patients is due to the new treatment and how much would have happened by chance or is due to standard treatment.
Placebo
Sometimes standard treatment is to ‘watch and wait’. This is also known as active monitoring or active surveillance and means that no treatment is given unless the cancer starts to progress or cause symptoms. In situations where there is no standard treatment to compare with the trial treatment, patients may be given a placebo. The placebo looks like the real drug but is inactive.
Placebos are also used in situations where a therapy is being added to the standard treatment to see whether this produces better results. One group of people will be given the standard treatment plus the trial therapy and one group of people will be given the standard treatment plus a placebo.
Blind trials
If you take part in a trial of a new drug you may not be told which treatment group you are in. This is called a blind trial. The medicine that all the people in the trial are given will look the same whether it is the new treatment, standard treatment or a placebo.
Some randomised trials are double blind, which means that neither you, nor the doctor treating you, know which treatment you are getting. Your doctor opens a specially coded treatment pack and only the trial organisers know which particular drug it contains.
Making a trial blind or double blind is intended to reduce bias. If you knew which treatment you were getting, it might influence how you felt. For example, knowing you were taking a new treatment might make you feel more positive, or more negative, and influence what you report to the researchers. Similarly, if the researchers knew that you were taking a new treatment for which they had high hopes this might affect how they judged your response to it. (In an emergency your doctor can always find out from the trial co-ordinators which treatment you are having, or the pharmacy department at the hospital will be able to break the code.)
Randomisation
All phase 3 and some phase 2 studies are randomised. This means that a computer will randomly allocate patients to the treatment groups in the trial. This is done so that each group has a similar mix of patients of different ages, sex and state of health.
If it were left to the researchers to decide who should get which treatment, they might be influenced by what they know about their patients.
Consciously or unconsciously they might put patients who were more or less likely to respond to a new treatment into a particular group. This would introduce bias, making the results unreliable.
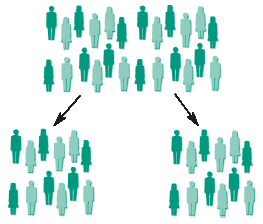
The benefit of randomisation is shown in the diagrams below.
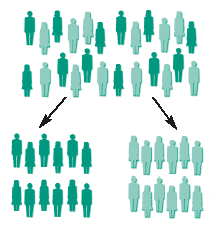
If doctors or patients choose the treatment, it is likely that people who are likely to do well will be in one group, leaving the others for the other group, so the two groups will be different. In that situation, if one group does better than the other, it will not be clear whether the difference is due to the treatment or because the groups were different.
If the treatment is allocated at random by a computer, the members of each group can be matched so that both groups are similar. If one group does better than the other group, it is likely to be because of the treatment, as the two groups are the same.
Page last modified: 02 November 2005






